William Daly

Emeritus Professor - - Natural Polymers -- Alumni Professor
Bachelor's Degree(s): Baldwin Wallace College, 1960
PhD: Polytechnic Institute of Brooklyn, 1964
PostDoc: University of Mainz, Germany
Phone: (225) 578-3237
Fax: (225) 578-3458
E-mail: [email protected]
Office: 712 Choppin Hall
Area of Interest
Our group concentrates on the synthesis of novel polymeric materials. Among our areas of interest are rigid rod polymers with unusual architecture, new polysaccharide derivatives, and asphalt polymer composites. Working with other members of the Macromolecular Research Group, we characterize all new materials and ascertain their potential applications. This research entails organic synthesis, monomer polymerization, polymer modification, graft copolymerization and preparation of polymer blends.
Our research on polysaccharides focuses on two areas: modification of polysaccharides to produce water soluble rigid rod or aggregating systems and conversion of chitosan to a soluble quaternary ammonium derivatives with biocidal properties. Techniques have been developed to convert carboxymethyl derivatives of cellulose, starch, chitin or chitosan into water-soluble aminoamide substrates suitable for further elaboration. For example we have produced quaternary ammonium adducts that can be used as cosmetic components and drug delivery adjuvants. The new cellulose derivatives enhance the viscosity of a formulation and exhibit biostatic properties. Recently we have developed a technique for elaborating cellulose with dendridic substutuents in a effort to enhance the concentration of substituents, and increase the rigidity of cellulose backbone. The dendrimer architecture has potential applications in catalysis and drug delivery. We are also interested in preparing cellulose-peptide graft copolymers with potential activity in calcium sequestration. These materials will duplicate the properties of natural chitin composites.
There is an unfilled need for new antimicrobial agents suitable for use as preservatives in personal care products as well as for new biocides to which bacterial strains have not yet developed resistance. We have observed that chitosan is readly converted to 3-trimethylammonium-2-hydroxypropyl-N-chitosan (CHI-Q188), N-Carboxy-methyl chitosan can be converted to a N',N'-dimethylammonium propyl carbamoyl-derivative and further modified by quaternization to produce a series of chitosan aminoamide quats.
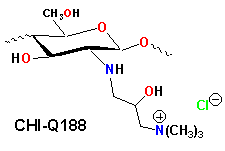
The antimicrobial activity of CHI-Q188 against Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa was determined using the minimum inhibitory concentration (MIC) test. The derivative exhibited biocidal activity at least an order of magnitude higher than previously reported chitosan antimicrobial agents. Derivatives of Quat-188 exhibited more moderate activity. The chitosan aminoamide quats also exhibited good antimicrobial activity. Efforts to elucidate the mechanism for this activity continue.
We are incorporating thiohydroxamic esters (Barton esters, BE) onto polymer backbones and using them to produce of graft copolymers efficiently. Asymmetric decomposition of BE’s promoted by either heat or light allows selective graft initiation by the bound acyloxy radical fragments. Efficient grafting of vinyl monomers from BE-modified polystyrene and poly(arylene ether sulfone) has been demonstrated. Utilization of BE’s as free radical sources allows for the formation of TEMPO unimers, which have been shown to initiate controlled radical polymerization. Appending these unimers to polymeric backbones allows for controlled radical graft copolymerization.
Cellulose derivatives can be converted to either the corresponding N-hydroxypyridine-2-thione ester (Barton ester) or carbonate. Either of these substituents could be photolyzed to produce graft copolymers of cellulose with styrene, acrylamide or N-isopropylacrylamide (NIPAAM). The styrene grafts were attached to cellulose backbone with labile carbonate linkages and terminated with thiopyridine groups generated by chain transfer to initiator. The NIPAAM copolymers exhibit a lower critical solution temperature; thermally reversible gels form upon cooling a solution to 32°C. Photolysis of the Barton carbonate in the presence of styrene and excess TEMPO produced a macroinitiator for controlled radical grafting of styrene to cellulose. The technique affords an opportunity to efficiently produce cellulose grafts of controlled lengths and to evaluate the influence of graft length on both the molecular properties and blending characteristics of the new materials.
Finally, we are exploring reinforcement of asphalt with modified polymeric wastes to develop an outlet for recycled polymers. We have found that slight modifications of polyolefins do improve the compatibility of the polymers with asphalt, as well as the compatibility of the various asphalt phases. Dynamic mechanical techniques are employed to predict the properties of asphalt polymer blends in asphalt cements. Efforts are directed to enhancing the properties most critical in producing blends with long term stability. Further work on this project, in conjunction with the Louisiana Transportation Research Center, will include the preparation, characterization and recycling of polymer/asphalt cements.
Awards & Honors
Alumni Professorship, 1999
Selected Publications
X. Wang, W. Daly, P. Russo and M. Ngu-Schwemlein. Synthesis of Paucidisperse Poly(y-benzyl-a, L-glutamate) Oligomers and Star Polymers with Rigid Arms. Biomacromolecules, 2001, 2, 1214-1219
W.H. Daly and T.S. Evenson. Grafting of Vinyl Polymers to Poly(aryl ether sulfone) Utilizing Barton Ester Intermediates and Nitroxide Mediation. Polymer, 2000, 41, 5063-5071
Former Ph.D. Students
Doris Culberson PhD, Solutia
Jack Davies PhD, Shakespeare Monofilament, Inc.
Soo Lee PhD, Changwon National University
Javier Macossay PhD, Professor, Facultad de Ciencias Quimicas at. Escue
Javier Nakamatsu PhD, Professor, Pontificia Universidad Catholica del Pe
Drew Poche PhD, Dow Research Labs
Zhaoyao Qiu PhD, DSM Copolymer
Xiaolan Wang PhD, OSCA